How we solved the hole in the ozone

The most striking thing was the solitude. As the airplane thundered across an unsettled sky toward Antarctica, I stared out the window at the landscape of shifting ice floes and dark ocean. There were no roads, no settlements, no structures of any kind—not even the occasional lonely ship on the south polar seas. As we approached the continent, the last rays of sunlight faded, replaced by a diffuse blue and purple twilight. That’s when I realized we really were on our way to the last place on Earth.
Just the year before, I’d been happily sitting at my desk in my warm and cozy office, where I studied stratospheric chemistry using computer models. The kingpin molecule in that chemistry is ozone, a highly reactive gas produced from oxygen that has unique abilities to absorb high-energy ultraviolet light. Earth’s fragile ozone shield stands between us and oblivion from the sun’s damaging rays and is what first allowed life to crawl out of the protective ocean and walk on land. A “layer” of ozone formed naturally in the stratosphere as oxygen evolved on Earth, some 10 to 30 miles over our heads.
But human activities can release a range of chemicals that eat away at it. The most damaging of these are compounds containing chlorine and bromine. Scientists had been expecting some ozone depletion due to human use of chlorofluorocarbon (CFC) chemicals—a small percentage, and a hundred years in the future, a “future” problem.
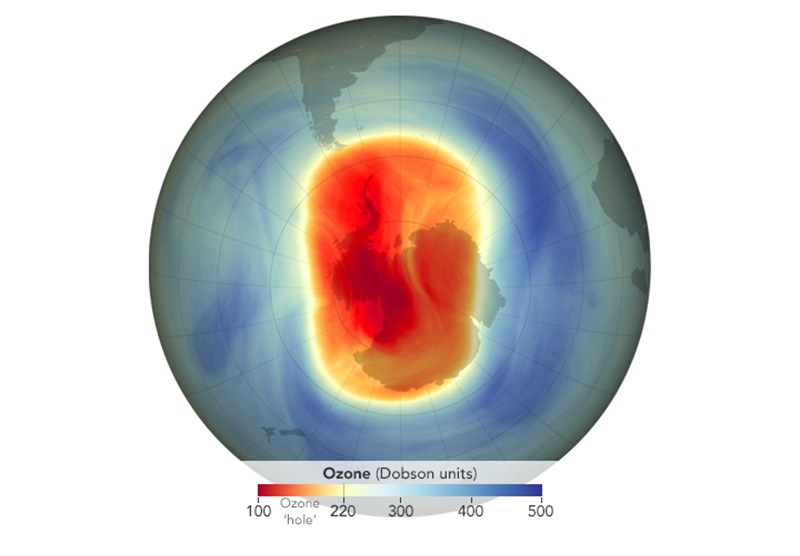
Then, in 1985, the British Antarctic Survey shocked the world by publishing a scientific paper declaring that an unexpected “hole” had formed in the ozone layer above their station. They reported a 50 percent loss of ozone, happening now, a wake-up call of outsized proportions. Scientists scrambled madly to figure out if the British measurements were correct, and if so, were humans the cause?
That’s what led me to that southbound flight in 1986. I was part of a team of researchers on our way to gather the data that would provide the first proof that chlorofluorocarbons were more effective at destroying ozone in the extremely cold Antarctic than anywhere else (including the Arctic, which is almost always warmer). The study of atmospheric chemistry had completely missed some critical extreme cold-temperature chemical reactions and therefore vastly underestimated the seriousness of the environmental problem.
The United States National Ozone Expedition (or NOzE as we called it) consisted of 16 scientists from four different research institutions. At age 30, I was the youngest and the only woman. Yet I was head project scientist, which made me the group’s spokesperson and occasional tough decision-maker. I enjoyed it more than I expected. I also gained a deep appreciation for how policy works, and the critical role of public opinion.
CFCs and other ozone-depleting chemicals have now been banned everywhere under the most successful global environmental treaty the world has ever known (so far), and humanity has already put the ozone hole on track to slowly heal itself. It would take me decades to understand how and why this miracle of environmental remediation happened.
That first flight to the Antarctic was not truly the beginning of my journey—I had researched the atmosphere for about a decade before I headed south, and my own work followed a long history of ozone research. In the late 19th century, scientists began to understand how the ozone layer shields us from an otherwise lethal sun.
If you have ever seen a sunbeam pass through a prism or crystal, you have seen how white light turns into a rainbow of color. These colors correspond to different wavelengths of light, with increasing energy from red to violet. Ultraviolet, a wavelength our eyes cannot see, has so much energy that it can damage life, including us.
In 1879, French scientist Marie Alfred Cornu measured different wavelengths of light coming from the sun and could not detect ultraviolet on the ground. He knew the sun must be emitting ultraviolet, but that some element in the atmosphere must be blocking it. Still, when he tested them, many of the usual suspects—oxygen, nitrogen, carbon dioxide, water vapor—all failed to block ultraviolet light. A few years later, British scientist Sir Walter Hartley determined that the molecule ozone—three oxygen atoms bonded together—could uniquely absorb some wavelengths of light, including ultraviolet. In 1924, another British scientist G.M.B. Dobson built the first scientific instrument to measure the protective amount of ozone above us. Research on understanding the formation and chemistry of stratospheric ozone continued for decades as an exercise in pure science.
In the early 1970s, researchers used a clever new technique to analyze air samples taken on a research ship that cruised from Britain to Antarctica. The new method, called electron capture gas chromatography, is exquisitely sensitive, and can detect the presence of gasses in amounts a million times smaller than those detected by previous approaches. Throughout the voyage, researchers measured the industrial chemical chlorofluorocarbon-11. There is no natural source for this chemical, but manufacturers loved to use it as a cheap and efficient propellant in spray cans. Its production had grown exponentially over the previous several decades.
Although almost all the spray cans were in the Northern Hemisphere, the researchers detected lots of the chemical in the Southern Hemisphere, too. The author of the paper reflected on the values measured all the way from the United Kingdom to the tip of Antarctica. This meant this CFC variety had to be nearly chemically inert—meaning it doesn’t react and therefore isn’t destroyed as it travels in the lower atmosphere.
This is profoundly different from the smog from Los Angeles, which may get as far as neighboring states but never all the way to the tropics, much less Antarctica. That’s because smog doesn’t live very long in the atmosphere. It can be rained out, and it reacts with vegetation and other surfaces. The paper noted that the chemical “constitutes no conceivable hazard” and suggested that measuring it might be a great way to learn about how winds move air around the world, a benign “tracer” of air motions.
Persistence is a property that should arouse uneasiness every time we encounter it in human impacts on our environment. We fear long-lived nuclear waste because we recognize that nuclear material is dangerous, so the idea that it hangs around essentially forever scares us. In a similar way, a large amount of the carbon dioxide from the coal, methane gas, and oil we burn is also going to be with us for thousands of years. If we’re going to do something irreversible on timescales of many decades (or more), we had better make doubly sure what we’re doing is safe. And we were doing absolutely nothing about the danger of the increasing amounts of persistent CFCs in the atmosphere.
The discovery of the ozone hole caused a shock wave through the scientific community worldwide when it was made public in the journal Nature in May of 1985. Many of my more senior colleagues were aghast that it had been published. If this was real, why hadn’t the satellites picked it up?
Solving that mystery didn’t take long. NASA scientists went back and rechecked their algorithms and data and reported within a few months that they saw the Antarctic ozone changes too, and they were covering most of the continent. In the view from space, you could easily see that a massive springtime ozone hole had formed. The satellite data could even be made into dramatic color video clips, in which you could see a swirling vortex with its ozone hole over the South Pole, rotating around like a hurricane viewed from space, a hurricane with a gaping hole instead of an eye.
As these images hit the TV and newspapers, the issue came alive for the public. If it proved to be due to CFCs, then it would be a scientific crisis that was perceptible to people. They realized that if anything like this ever occurred over their own heads, it would be deeply personal.

The ozone hole also had the allure of scientific mystery. The best experts could not be sure why it was happening, but the evidence was clear: It was real. It was many times worse than anyone’s model predictions for CFCs.
We needed more data if this mystery was going to be solved. The process of getting that data would also capture the public imagination, because Antarctica exemplifies the great unknown and great untamed, a place where scientific heroes go to extremes for the sake of knowledge. This problem wasn’t something that one or two scientists would take on as individuals. It was going to take multiple measurements done by teams, as well as other teams working in the laboratory or on increasingly sophisticated models.
And the world would be watching, so we needed to do it fast and we needed to do it well. Yikes. But from a scientific point of view, it was the pinnacle of excitement and fun. There is nothing more fascinating to me than attacking a scientific mystery, and what could be more fantastic than one involving the coldest, remotest place on Earth?
Antarctica is also the coldest place up in the stratosphere. The stratosphere is normally too dry for cloud particles to form, but under the extreme cold of Antarctica, what are known as polar stratospheric clouds can form.
But they were considered a curiosity—not a player in the chemical reactions of the atmosphere. Gaseous chlorofluorocarbons break down in the high-intensity light of the stratosphere and are converted mainly to hydrochloric acid and chlorine nitrate gas. As long as the chlorine stays there, ozone is safe. And these two things don’t react together at all in the gas phase.
But I began to think about what could happen on the surfaces of those icy cold clouds. Surfaces can change chemistry profoundly; that’s why our gasoline-powered cars have catalytic converters whose surfaces transform our auto exhaust into less dangerous compounds. I reasoned that hydrochloric acid and chlorine nitrate could come together and react on those polar cloud particle surfaces, liberating ozone-destroying chlorine back to the gas phase. That, along with the need for a little bit of sunlight (to drive additional reactions) was the essence of my idea.
Many of my colleagues would view our study as an outrageous idea, because nearly everyone believed the then-conventional wisdom that only reactions between gas molecules could matter in the stratosphere. A few colleagues delighted in telling me that this surface chemistry couldn’t possibly work, with one saying “these [cloud] particles don’t behave like swimming pools” where reactions could happen. But in the end, it turned out that’s exactly how many of the particles can behave. Everyone’s prior work had involved gas molecule reactions only—and the chemistry of surfaces was why science had missed predicting the Antarctic ozone hole before it happened.
It was not the whole story by any means, but surface chlorine chemistry on these clouds would turn out to be the key first step in producing the ozone hole, and remains essential to modeling the stratosphere today. The reason why an ozone hole wasn’t forming in the Arctic is simply because it is generally much warmer than the Antarctic, although there have been a couple of extremely cold Arctic years recently when depletion there rivals that in the Antarctic.
I presented the work to colleagues at a scientific meeting in that same month. I remember well the skepticism with which my presentation was met, with lots of hard questions. But hard questions are what science is all about, and they make our work stronger. It needs to be strong if people are going to make industrial decisions or base policies on it.
Protecting the ozone layer through the 1987 Montreal Protocol is rightly called the world’s greatest international environmental success story, celebrated globally and held up as evidence of what people can achieve in managing environmental risk. We were helped when solving the ozone problem by an ideal confluence of what I call the “three P’s”: It had deeply personal health impacts, its science was readily perceptible to non-experts, and solutions were eminently practical.
Preventing ozone depletion had the tremendous benefit of a powerful kickstart caused by consumer action to turn away from CFCs in spray cans in the U.S., a personal choice that destroyed the market for American CFCs and made our producers eager to look at alternatives. It was an easy thing to do, and it made people feel empowered and interested.
Can progress still happen when it depends not on personal choices but policies? Sure. Getting rid of CFCs in refrigeration and air conditioning was not something the individual consumer could do, beyond limited personal choices. But technology-steering policies under the protocol inspired the required innovation to find those solutions and make them cheap enough to be practical.
The discovery of the Antarctic ozone hole stunned the entire world and made the ozone issue a “hot crisis.” People are much better at solving hot crises than they are at dealing with slow ones. The public’s fascination kept scientists energized and politicians well motivated to act. Scientists grew accustomed to the awesome power of teamwork: in field experiments to the ends of the Earth and most important, in international assessment reports. We stopped working as lone wolves and became an effective pack, aware that as a group we could serve the world better than any of us could on our own.
The Antarctic ozone hole does vary from year to year, buffeted by things like volcanic clouds and wildfire smoke that can lead to a long-lasting hole for a year or two, until those extra particles disperse. But signs of healing the damage we’ve caused are already clear in the longer run. Can we keep it up? We’ll always need to be alert to new threats—whether, potentially, the metal particles shed by satellites or increasing numbers of pleasure trips to space—so stay tuned.
Reprinted with permission from Solvable: How We Healed the Earth, and How We Can Do It Again by Susan Solomon, published by the University of Chicago Press. © 2024 by Susan Solomon.
This article originally appeared on Nautilus, a science and culture magazine for curious readers. Sign up for the Nautilus newsletter.