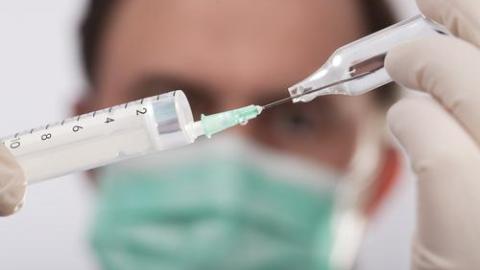
Canada to Begin Testing HIV Vaccine
What’s the Latest Development?
A team of Canadian researchers has been given approval by the US FDA to begin testing their HIV vaccine on humans. Tests will begin in January with a group of 40 HIV-positive patients to test the vaccine’s safety. Leader of the team, Dr. Chil-Yong Kang said: “The SAV001 vaccine, administered as an injection, has already gone through preliminary toxicology tests on animals. It didn’t show any adverse effects or safety concerns and can be produced in large quantities.”
What’s the Big Idea?
An HIV vaccine could be ready in five years time, say the researchers at the University of Western Ontario. Tests will begin on HIV-positive patients to determine the vaccine’s safety, then move to HIV-negative patients considered to be at high risk of contracting the virus, “including hemophiliacs, injection drug users, sex trade workers and those in the gay community with multiple sexual partners.” Trials for the first phase of study are scheduled to begin in January of next year.
Photo credit: shutterstock.com