We might have Alzheimer’s all wrong
- The amyloid cascade hypothesis has dominated Alzheimer’s disease research for over three decades. However, growing evidence — including possibly fraudulent research — challenges its validity.
- According to an alternative hypothesis, brain cells undergo metabolic reprogramming, which causes healthy neurons to starve while damaged neurons survive.
- Evidence is emerging that our understanding of Alzheimer’s is incomplete at best and completely misguided at worst. The paradigm is shifting.
In 1906, Alois Alzheimer, a German psychiatrist and pathologist, dissected the brain of a woman who had died of an unusual mental illness. Her symptoms included memory loss, language problems, and unpredictable behavior, and Alzheimer’s examination revealed abnormal clumps in her brain tissue. Over the next century, scientists came to believe that these masses were the cause of the symptoms and searched for an explanation of their origin. Finally, in 1991, two neuroscientists, John Hardy and David Allsop at St. Mary’s Hospital Medical School, presented an answer that would dominate Alzheimer’s disease research and guide thousands of scientific studies. But what if their explanation was wrong?
Rise and fall of the amyloid hypothesis
Hardy and Allsop termed their explanation the amyloid cascade hypothesis. It posits that Alzheimer’s disease develops due to biochemical changes in the brain. Toxic molecules (specifically, misfolded proteins) build up and clump together, forming aggregates in the brain. These aggregates cause neuron death, which ultimately results in cognitive decline.
Over the last decade, however, a growing number of scientists have begun to question the validity of the hypothesis. Although the misfolded proteins are always present in the brains of patients with Alzheimer’s, they are also found in about 30% of people who display no clinical manifestations of dementia. Additionally, drugs that target the misfolded proteins are ineffective at treating the disease. In 2021, scientists at the University of California-San Francisco analyzed the results from 14 anti-amyloid drug clinical trials. Their analysis revealed that removing the protein plaques does not improve the cognitive functions of patients with Alzheimer’s. Even worse, an exposé in the journal Science reveals that some key research in the field appears to have been fabricated.
If the amyloid hypothesis is wrong, is there a better explanation? In addition to creating clumps of toxic proteins, the brains of patients with Alzheimer’s disease change their bioenergetic signature. In other words, their brain changes how it produces and utilizes energy, and some scientists believe this is the underlying cause of Alzheimer’s.
Is Alzheimer’s a metabolic disease?
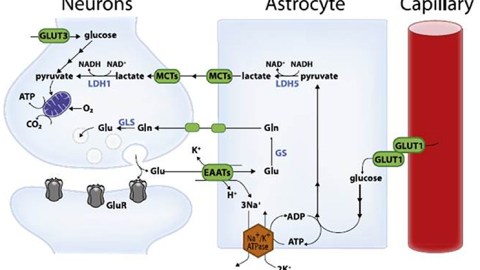
High-level cognitive abilities and complex behaviors require a tremendous amount of energy. Consequently, the brain has an insatiable sweet tooth. It consumes 25% of the body’s glucose supply, despite only constituting about 2% of the body’s total weight.
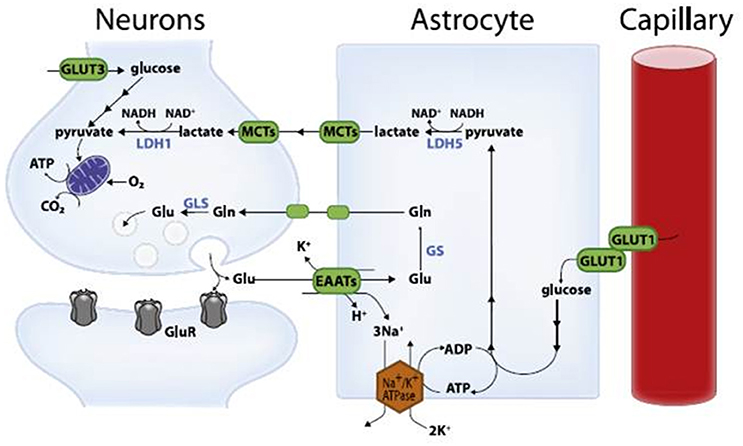
Surprisingly, the neurons responsible for these complicated functions are not sugar addicts; the cells that support them, called astrocytes, are. Astrocytes are particularly well-equipped to snatch up glucose and metabolize it through a process called glycolysis. This process is relatively slow and doesn’t yield much energy. It does, however, convert the six-carbon sugar into a three-carbon molecule called lactate, which is the fuel of choice for neurons. Neurons then take up lactate, convert it into a different molecule (pyruvate), and rush it to the mitochondria. In that powerhouse, a metabolic process called oxidative phosphorylation uses oxygen to yield a tremendous amount of energy.
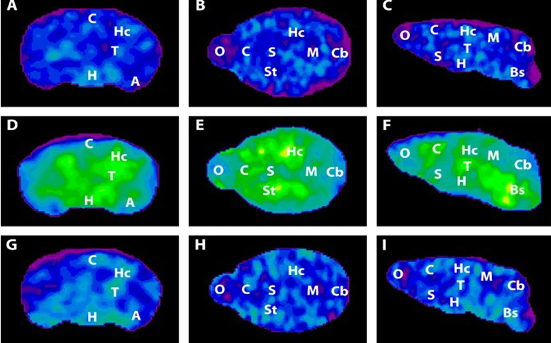
However, some neuronal mitochondria show signs of metabolic defects and bioenergetic dysfunction during the earliest stages of Alzheimer’s, according to previous studies. Consequently, each unit of lactate provided to a neuron yields less energy. To keep up with the energy demand, neurons with damaged mitochondria reprogram their metabolic processes. Essentially, their metabolism is pushed into overdrive, and they begin depleting the local environment of lactate.
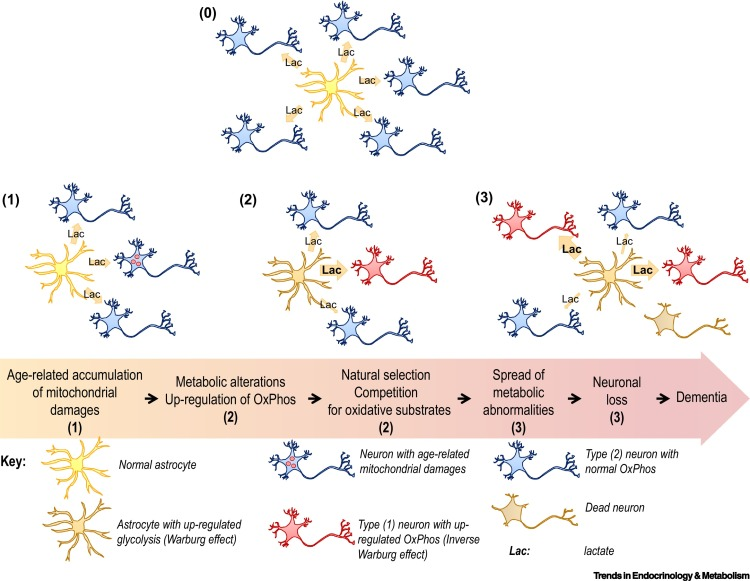
Astrocytes also undergo metabolic reprogramming, kicking their metabolism into overdrive to produce more lactate. This saves neurons with damaged mitochondria from starvation. Healthy neurons, however, are not so lucky. When nutrients are scarce, neurons with higher metabolic rates have a selective advantage. Essentially, when there isn’t much food, the slow eaters will starve first — and healthy neurons are slow eaters. Over time, healthy neurons die, the neural environment degrades, and dementia ensues.
Alzheimer’s may not be entirely age-related
Alzheimer’s is considered an age-related disease (except for early-onset Alzheimer’s disease). In 2017, however, a team of scientists at Harvard studying skin cells made a discovery that partially challenged this idea.
They found that skin cells from patients with Alzheimer’s also undergo changes in bioenergetic metabolism similar to neurons. Interestingly, old healthy people did not experience these changes, whereas patients with Alzheimer’s disease did. In other words, the mitochondrial damage and dysfunction in skin cells were not due to aging; they were inherent to the individual. Following this discovery, the team sought to determine if metabolic dysfunction occurred before brain cells developed. They isolated neural progenitor cells, the earliest progenitors from which all brain cell types originate, and discovered that even the progenitors exhibited altered bioenergetic features.
Evidence is emerging that our understanding of Alzheimer’s is incomplete at best and completely misguided at worst. While Alzheimer’s is clearly linked to age, it appears that abnormal cellular energy management inherent to particular individuals plays a substantial role in the development of disease. The paradigm is shifting.