Scientists stole a mutation from cancer and used it to kill tumors
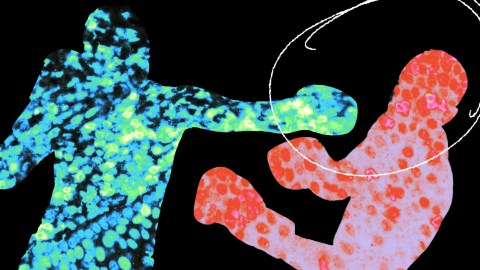
- CAR-T cell therapies engineer T-cells to fight cancer.
- However, CAR-T cells don't work well against solid tumors because the cancer renders its microenvironment inhospitable for T-cells.
- By inserting a cancer-cell mutation into CAR-T cells, researchers have managed to kill solid tumors in mice.
By inserting a mutation found in cancer cells into CAR-T cells — a powerful weapon against blood cancers — scientists from UC San Francisco and Northwestern University were able to get the cells to kill solid tumors in mice.
“We’ve used the mutations that give cancer cells their staying power to engineer what we call a ‘Judo T-cell therapy’ that can survive and thrive in the harsh conditions that tumors create,” said co-author Kole Roybal.
CAR-T cells: CAR-T cell therapy starts with doctors extracting T cells — a type of white blood cell — from a cancer patient’s blood. They then engineer the T cells to display proteins called “chimeric antigen receptors” (CARs) that bind to cancer cells.
The CAR-T cells are then multiplied and infused back into the patient where they help the immune system fight the cancer.
Natural T-cell function isn’t good enough. We need to explore the extremes of T-cell function.
Kole Roybal
The challenge: The FDA has approved several CAR-T cell therapies for use against blood cancers, but getting the therapies to work against solid tumors, which account for 90% of adult cancer cases, has been a challenge.
Not only is the microenvironment around a tumor inhospitable to T cells — even specially engineered ones — tumor cells can also trick immune cells into helping them survive.
The idea: Because cancer cells are super resilient, the UCSF and Northwestern team hypothesized that there might be some mutations in cancerous T cells — the origin of certain lymphomas — that they could use to supercharge CAR-T cells.
“Natural T-cell function isn’t good enough,” Roybal told Nature. “We need to explore the extremes of T-cell function.”
The study: An analysis of lymphoma patients’ cancerous T-cells led the researchers to 71 mutations. By creating CAR-T cells with these various mutations, they discovered that one mutation in particular made the CAR-T cell therapy 100 times more potent against cancer cells.
It didn’t just work against blood cancers, either — in tests on mice, it was able to destroy solid skin, lung, and stomach tumors.
“We don’t know exactly the mechanism, but it seems to get in better, it seems to thrive better in the [tumor] microenvironment, it seems to proliferate better, and it seems to kill better,” co-author Jaehyuk Choi told the Nature Podcast.
There’s so much to learn from nature about how we can enhance these cells and tailor them to different types of diseases.
Kole Roybal
The concerns: People treated with CAR-T cell therapies have, in rare instances, developed secondary cancers. This makes the idea of inserting a mutation found in cancerous cells into CAR-T cells seem particularly risky.
The animal studies suggest the new approach is safe, but the researchers are still looking for ways to ensure they wouldn’t be inadvertently giving someone lymphoma while treating their solid tumor — some kind of molecular “switch” that could be used to deactivate the cells is one possibility.
Looking ahead: The researchers have founded a startup, Moonlight Bio, to continue development of their supercharged CAR-T cell therapy. Their first target is lung cancer, and they hope to have a therapy ready for clinical trials within a few years.
They also plan to continue hunting for mutations that could make the therapy even more robust.
“We see this as the starting point,” said Roybal. “There’s so much to learn from nature about how we can enhance these cells and tailor them to different types of diseases.”
This article was originally published by our sister site, Freethink.