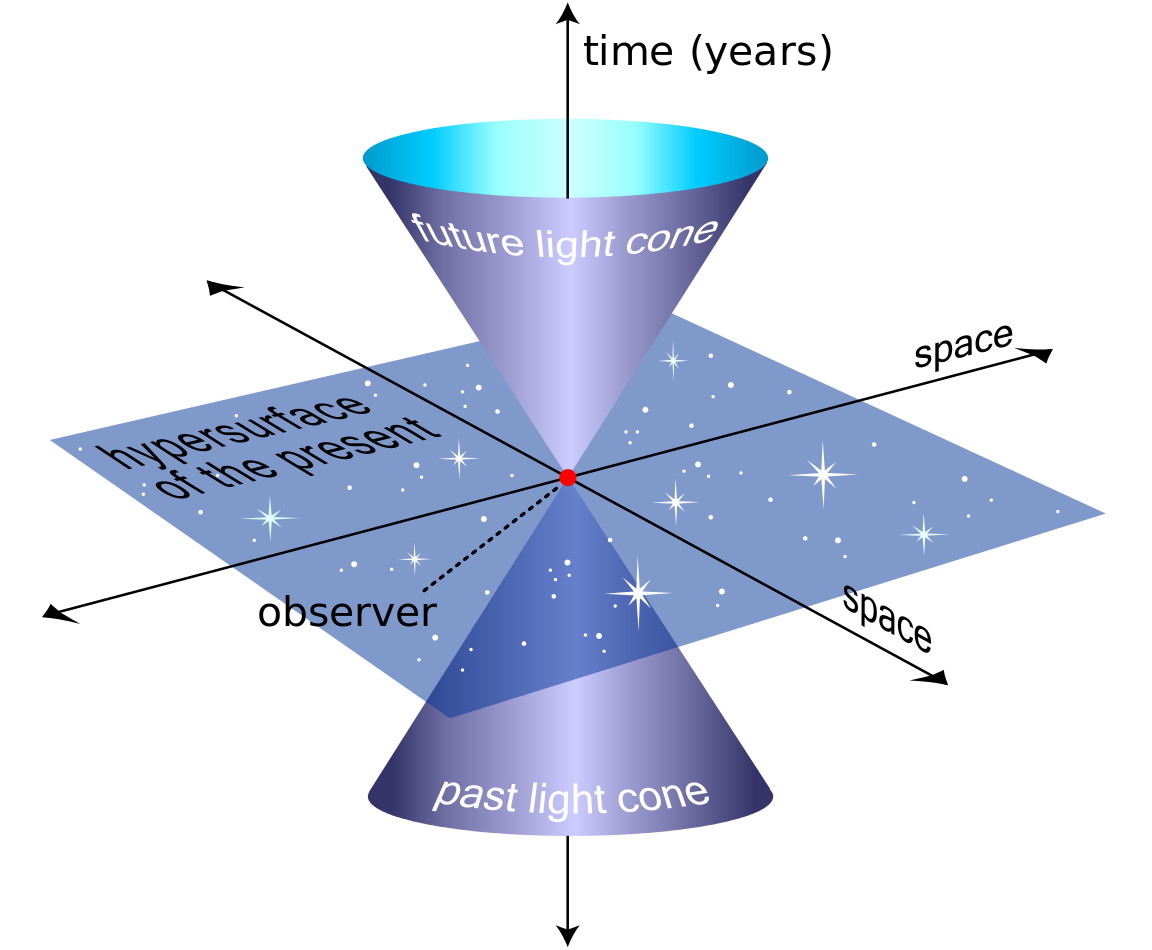
A clock beats inside the heart of every atom

- Across the centuries, the pace of cultural evolution can often be tied to the machines available for measuring time.
- The advent of industry required us to build clocks that could measure time down to the second. But the gears and springs in a mechanical timepiece wear down and lose their accuracy.
- Our contemporary ability to tell time down to the nanosecond is key to all of the technology we rely on today.
Measuring time has always been fundamental for humans, and different societies across history have developed different ways of tracking it. As I explored some years ago in my book About Time: Cosmology and Culture at the Twilight of the Big Bang, the pace of cultural evolution can often be tied to the machines available for measuring time. Almost every new timekeeping technology has ushered in new societal arrangements. What is especially remarkable about the technology we use in the modern world is that it all rests on physics operating at the atomic scale.
From the position of the sun to that of electrons
In the pre-industrial age, people only needed to measure years and months to a fair amount of accuracy. The position of the sun in the sky was good enough to break up the day. Timing at the level of fractions of a second was simply not needed.
Eventually, modern industry arose. Fast-moving machines came to dominate human activity, and clocks required hands that could measure seconds. In the current era of digital technology, the timing of electronic circuitry means that millionths or billionths of a second actually matter. None of the high-tech stuff we need, from our phones to our cars, can be controlled or manipulated if we cannot keep close track of it. To make technology work, we need clocks that are faster than the timing of the machines we need to control. For today’s technology, that means we must be able to measure seconds, milliseconds, or even nanoseconds with astonishing accuracy.
Every timekeeping device works via a version of a pendulum. Something must swing back and forth to beat out a basic unit of time. Mechanical clocks used gears and springs. But metal changes shape as it heats or cools, and friction wears down mechanical parts. All of this limits the accuracy of these timekeeping machines. As the speed of human culture climbed higher, it demanded a kind of hyper-fast pendulum that would never wear down.
Luckily, that is what scientists found hiding inside the heart of each atom.
Every atom absorbs and emits electromagnetic radiation at special frequencies. These frequencies (and their related wavelengths) change based on the element. Expose an atom of hydrogen to the full spectrum of optical light, and it will absorb only a few frequencies (colors). Other frequencies remain untouched. In the early decades of the 20th century, the field of quantum mechanics explained this strange behavior. Quantum theory showed how the transitioning of electrons defines the interaction of light and matter. The electrons jump from one orbit around their atom’s nucleus, to another.
Atomic time makes the best clocks
Absorption entails an electron jumping to a more energetic orbit as a light particle, or photon, is captured. Emission is the opposite — an electron jumps to a lower orbit, releasing energy as a photon is emitted. Using quantum mechanics, physicists learned how to precisely predict the frequencies of absorption and emission of all atoms, ions, and molecules.
Though no one knew it at the time, these quantum jumps would make for a new kind of clock. Frequency is nothing but inverse time (1/seconds). This means extremely accurate measurements of the transition frequency of an atom or molecule can transcribe a precise measurement of time.
In World War II, the development of radar allowed waves in the microwave region of the electromagnetic spectrum to be used in photon-atom interaction experiments. This led to the first atomic clock, which was based on ammonia molecules and their microwave frequency transitions.
Cesium atoms later became the preferred tool for time measurement, and in 1967 the second was formally defined as exactly 9,192,631,770 cycles of the cesium atom’s transition frequency. Modern atomic clocks are now so precise that their accuracy is measured in terms of gaining or losing nanoseconds per day.
None of the modern miracles that facilitate our daily lives would work without these “pendula” inside atoms. From the GPS satellites sending and receiving signals across the globe, to the tiny switches inside your cell phone, it is the most basic aspect of modern physics — quantum jumps — that allows such delicate filigrees of time.