Why blue LEDs are worth a Nobel Prize
It might not sound impressive, but the physics behind it — and the power of its applications — are literally world-changing.
Image credit: Orphek LEDs, via http://orphek.com/about/aquarium-led-lighting/.
“Everyone must leave something behind when he dies… Something your hand touched some way so your soul has somewhere to go when you die… It doesn’t matter what you do, so long as you change something from the way it was before you touched it into something that’s like you after you take your hands away.” –Ray Bradbury
Every year around this time, the biggest awards in all of science — the Nobel Prizes — are given out. And every year, the same arguments take place:
- People in one sub-field claiming that their area’s favorite/most impactful advances should have been honored instead of the one that was chosen.
- People within a particular sub-field complaining that other individuals should have been honored along with/instead of the ones chosen.
- And finally, by people who don’t think the research chosen to be honored was “Nobel-worthy.”
At least when it comes to the Nobel Prize in physics, there are literally so many good choices that I can’t find even one in the Nobel’s 114 year history that falls in that last category. And that includes this year’s winners, too.

The three men above — Akasaki, Nakamura and Amano — were awarded the Nobel Prize in Physics for 2014 for their discovery and development of… the blue LED.
That’s right: for a blue, light-emitting-diode. Like the kind you’ll see on your street’s Christmas lights in oh, about four weeks.

Seems pretty frivolous, doesn’t it? Except the applications are huge, and the physics behind it is, well, kind of revolutionary. There were two great steps that happened prior to this, and this is the third great leap forward in this field. What it’s opened up is much more than most people realize when they think of how humdrum “Blue LEDs” sounds.
In particular, here are the three great steps:
- The invention of the diode.
- The invention of the light-emitting diode, or LED.
- The development of LEDs that cover all of the visible light spectrum.
Let’s go through each of these, and what new things they brought to the world with each step forward.

1.) The invention of the diode. It’s easy to forget that a mere 150 years ago — when our grandparents’ grandparents were in the primes of their lives — electricity was a rare thing to come across. All the basic electronic components, things like resistors, capacitors, inductors and light bulbs were first being discovered and utilized. And one of the things that came out of that early period of electrical experimentation was the discovery of the diode: a material that allowed electricity to flow in one direction but not the reverse!
It’s a little overly simplistic, but you can imagine a diode the same way you’d imagine a funnel: a cone-shaped object with a hole (or spout) at the bottom.

If you have something flowing through the fat end of the funnel, it flows easily through the other end: there’s practically no resistance. But if you flip the funnel, and try to flow that same material through the skinny end, there’s practically no flow, because the resistance is huge!
Diodes are basically the same thing, except for electricity instead of flowing fluid. You can think of electricity as being made up of “electrons,” which move and carry charge, and “holes,” which are the places that the electrons can flow to. (Although, mathematically, the movement and transport of “holes” is just as valid as the movement and transport of “electrons.” This will be useful to keep in mind, even though in real life it’s only the electrons that move.) The ability to allow electricity to flow only in one direction is incredibly useful for a number of applications, and just a few types of diodes, vacuum tube diodes and solid-state (e.g., crystalline) diodes gave rise very quickly to a number of huge applications: the wireless telegraph, precision radio detectors, the Fleming valve, AC/DC converters, and a little later, the first guitar amplifiers.

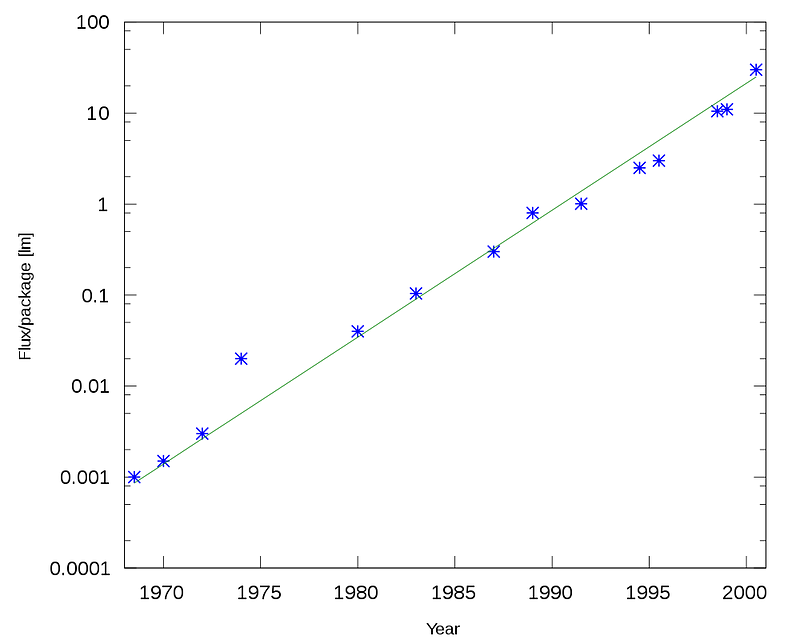
2.) The Light-Emitting Diode (LED). Now imagine the same funnel-shaped structure, but when the electric current flows in the direction it’s allowed to flow in, and the “electrons” flow into the “holes,” a certain amount of energy is released. Because energy of a particular frequency corresponds to electromagnetic radiation of a particular wavelength, these types of diodes can emit light. LEDs are pretty much all solid-state diodes, meaning that they’re made of semiconductor materials, but when electricity passes in the forward direction, the material emits light at the particular frequencies that the material in question allows, dependent on what’s known as the “band gap,” or the energy difference that’s released when the electron goes in its hole.

The phenomenon of light being emitted when you pass electricity through something is common to many different materials, and is known as electroluminescence. You might not realize it, but electroluminescent materials are everywhere, and aren’t just limited to diodes. When you think about it, a material that emits a narrow wavelength-band of light when you pass electricity through it has practically limitless applications!

But light-emitting diodes are special among electroluminescent materials, because of the ability to only allow current flow in one direction! This is a much harder problem than mere electroluminescence, and so if you want to create not just an electroluminescent material, but an electroluminescent diode, you have to find the right semiconductor material with the right band-gap in energy to produce visible light of the right frequency and of the right brightness.
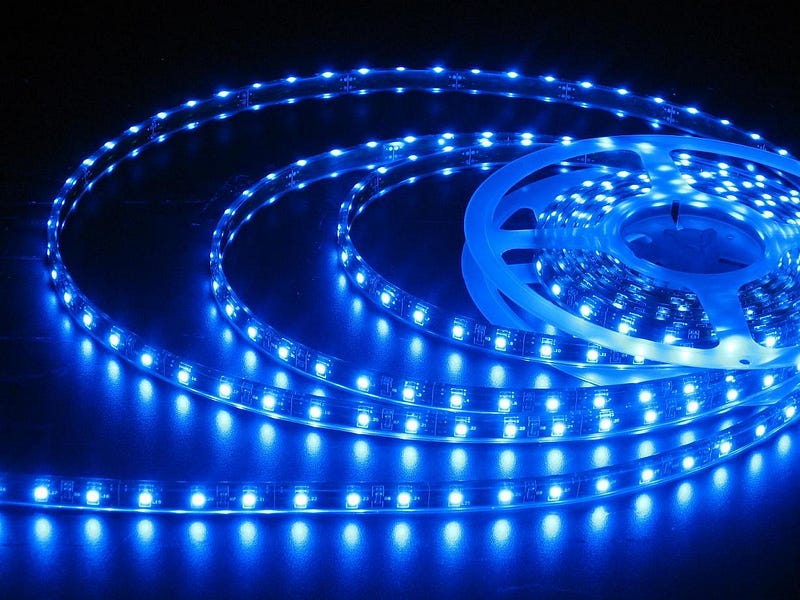
The first LEDs were in the infrared, but it was in the early 1960s that the first visible light LEDs were invented: in the red. Over the next few years, new materials were discovered that allowed the brightness to be increased, resulting in the first widespread application: red LED displays.

When I state that brightnesses have increased, this is something that’s happened incredibly fast over a relatively short period of time. The first LEDs were barely perceptible to human vision, yet have increased in brightness by more than a factor of 100,000 since their discovery just over 50 years ago.
But if we wanted to replace our incandescent lights, our LCDs, our cathode ray tubes or any other full-spectrum light needs with LEDs, we’d need to be able to cover the full spectrum of visible light. And what that would mean for LEDs is the discovery of materials that could produce all the necessary colors: in particular, red, green and blue, which could produce all the colors if added together in the right amounts.
While materials that produced red, yellow and green LEDs were easy to come by, blue was the hard problem. For over 30 years, from 1962 until 1994, there was no such thing as a blue LED light.

3.) The development of a blue LED. At the risk of oversimplifying all of solid-state physics, the key to building any LED is to find the right material to serve as the semiconductor “die” in the diode. What’s needed is a band gap that drops a specific amount in voltage and releases a specific wavelength of light when the electron finds its hole. As we covered earlier, while many different types of materials emitted red, yellow and green light, a blue LED was elusive.
The key breakthrough came because of materials advances using Gallium Nitride (GaN), and by nucleating that material in particular on sapphire substrates, allowing scientists to finally create the p-n junction (the interface between the two fundamental types of semiconductor) critical for constructing a diode. As it turned out, adding Indium Nitride to the Gallium Nitride mix (known as InGaN) allowed for the LED to not only emit blue light, but to emit it at an incredible brightness.
These key developments were made in 1994, with Akasaki and Amano figuring out how to nucleate the GaN onto sapphire substrate, and Nakamura coming up with the final InGaN step. They not only created the first blue LED, but this advance quickly led to the development of brilliant white LEDs, and to extremely small, low-powered RGB LEDs that could create backlit screens using tiny amounts of power. Pretty much every smartphone screen in existence takes advantage of this LED technology.

It might not have the fundamental importance that the Higgs boson does; it might not revolutionize our understanding of the Universe like the discovery of dark energy did; and it might, in fact, be so mundane that we already take it for granted. But it’s some amazing science that’s already led to some fantastic applications, and has the short-term potential to reduce the amount of energy that humans spend on lighting alone from 20% of our world’s energy use all the way down to just 4%.
Not bad for putting a few atoms in just the right place.
Leave your (congratulatory?) comments at the Starts With A Bang forum here!